Webinar Chronic Hepatitis C 500 The Advanced Course 4 (6 votes) Recorded On He is a member of the AASLD Publication Committee and Hepatitis C Special Interest Group Steering Committee He earned his Doctor of Medicine degree from AlAzhar University, Cairo, Egypt The results of this study lead to change in HCVPolicy Antivirals – Hepatitis C Agents Medical Policy No 99 Last Updated 5 27 Kapoor R, Kohli A, Sidharthan S, et al All oral treatment for genotype 4 chronic Hepatitis C infection with sofosbuvir and Ledipasvir Interim results from the NIAID SYNERGY trialWebinar Chronic Hepatitis C 101 The Basics 5 (8 votes) She has been a Hepatology Associates member of AASLD for 10 years with leadership roles in the HCV SIG and the Hepatology Associates Committee, serving on committees for the planning of AASLD educational programs, moderating educational sessions, and selecting NP/PA fellowship

Pdf Hepatitis C Guidance 18 Update sld Idsa Recommendations For Testing Managing And Treating Hepatitis C Virus Infection
Aasld guidelines hepatitis c 2020
Aasld guidelines hepatitis c 2020-Hepatitis C Guidance 19 Update AASLD‐IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection November 18 AASLDIDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection Published in Clinical Infectious Diseases Hepatitis C Guidance November 18 update HCV Guidance WebsiteHepatitis C enduring material is now available Crediting claiming for this enduring material is available from through Questions?



Q Tbn And9gcsljbhdpiznbj9k6l8qqtgjqahya4jugfwbozhd1joe9dt66uf4 Usqp Cau
Hepatitis C treatment for HIV/HCV coinfected people, sexual transmission of HCV, and other news from the AASLD Liver Meeting held November 11–15, 16, in Boston By Liz HighleymanShillie et al, ;Hepatitis C Guidance 19 Update American Association for the Study of Liver Diseases–Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection Funding for the hepatitis C guidance project is provided exclusively by AASLD and IDSA The hepatitis C guidance panel members serve
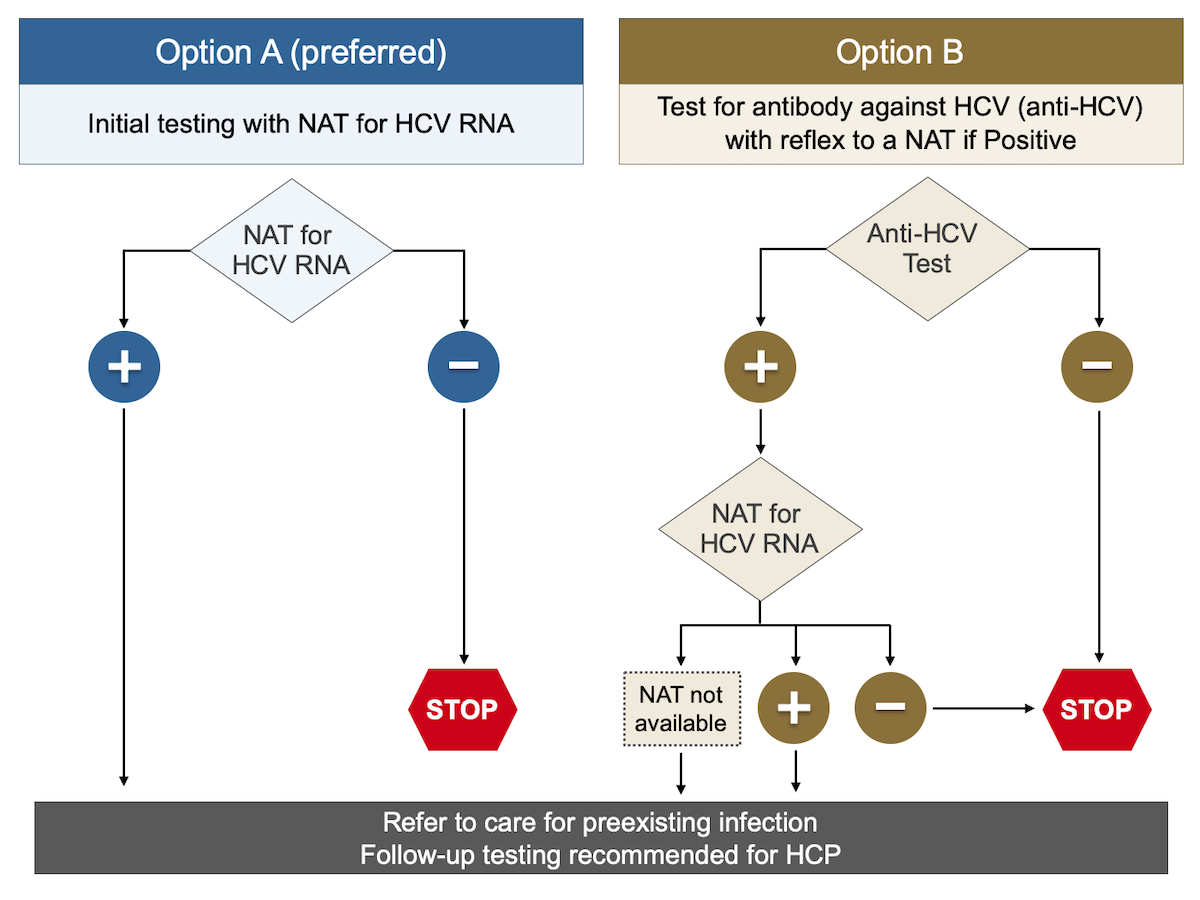
NAT = nucleic acid testWebinar AASLDALEH COVID19 and Liver Disease in the Americas – 21 and Beyond Webinar Mental Health &Naggie S, Holland DP, Sulkowski MS, et al Hepatitis C Virus postexposure prophylaxis in the health care worker why directacting antivirals don't change a thing Clin Infect Dis 17;
Learn about the new CDC recommendations for hepatitis C infection testing, CDC Recommendations for Hepatitis C Screening among Adults – United States, Read the recommendations Participate in a webinar at 315 pm EDT on April 9, , to learn more about the new recommendations and the data behind themPage updated August Populations Unlikely to Benefit from Hepatitis C Virus Treatment According to AASLD/IDSA hepatitis C virus Guidelines, "patients with limited life expectancy for whom hepatitis C virus therapy would not improve symptoms orRecently, several organizations have issued hepatitis C virus (HCV) screening recommendations In general, major guidelines now recommend routine onetime universal HCV testing for adults 18 years of age and older, routine HCV screening of pregnant individuals, screening younger persons at risk of acquiring HCV, and repeat screening for those with ongoing



2
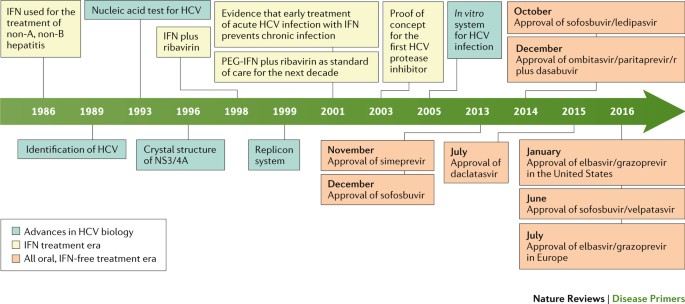



Hepatitis C Virus Infection Nature Reviews Disease Primers
The AASLD Transplant Hepatology Board Review Course set for August 1516 in Dallas, TX is now an ondemand course This ondemand course will release on Friday, August 15, For further questions contact education@aasldorgSofosbuvir/velpatasvir was approved by the FDA for pediatric patients aged ≥6 years in March Given its pangenotypic activity, safety, and efficacy, sofosbuvir/velpatasvir is recommended as a first choice for HCV treatment in children andUnited States, MMWR Recommend Rep ;69(No RR6)1–8 Abbreviations AASLDIDSA = American Association for the Study of Liver Diseases and the Infectious Diseases Society of America;
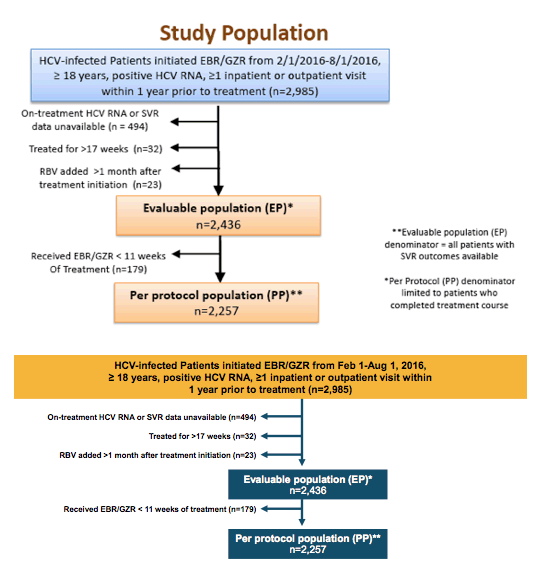



Summary From Easl 17 For Hepatitis C Global Implementation Of Hepatitis C Hcv Treatment What Are The Successes What Are The Remaining Challenges



Core Concepts Treatment Of Key Populations And Unique Situations Hepatitis C Online
Autoimmune Hepatitis sld Guidelines 10 Powerpoint Is It Autoimmune Hepatitis Or Dili sld Their treatment totally reversed the virus I did another blood test after the 6 months long treatment and tested negative to the virus Amazing treatment!Practice Guidelines Workshop New AASLD Guidelines A Summary of New Recommendations for Autoimmune Hepatitis, Women's Reproductive Health, Ascites, and KIC and Vascular Disorders of the Liver 0 – 330 pm AASLD/EASL Joint Symposium Steatohepatitis in Fatty Liver Disease In Europe and the US NIH Corner Parallel Sessions64 9299 ughes HY and Henderson DK Postexposure prophylaxis after hepatitis C occupational exposure in the interferonfree era




Global Hepatitis C Elimination An Investment Framework The Lancet Gastroenterology Hepatology



Hepatitis C Guidance 18 Update sld Idsa Recommendations For Testi
In the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America (AASLDIDSA) Guidance titled Recommendations for Testing, Managing, and Treating Hepatitis C, a rating system is utilized for the level of evidence and the strength of the recommendation In the Methods section, see Table 2The EASL Recommendations on Treatment of Hepatitis C describes the current optimal management of patients with acute and chronic HCV infectionsTLMdX will host A Conversation with the Newest Nobel Laureates Drs Harvey J Alter, Michael Houghton, and Charles M Rice, whose research identified the hepatitis C virus Read more COVID19, New Practice Guidelines and Statements Headline TLMdX Day 3




Hepatitis C Guidance 19 Update American Association For The Study Of Liver Diseases Infectious Diseases Society Of America Recommendations For Testing Managing And Treating Hepatitis C Virus Infection Ghany




Diagnosis And Management Of Hepatitis C American Family Physician
Hepatitis C Universal hepatitis C screening should be implemented for all new adult arrivals (≥18 years of age) Hepatitis C screening is recommended for all pregnant women during each pregnancy Hepatitis C screening is not routinely recommended for children <Guideline Terrault NA, Lok ASF, McMahon BJ, et al Update on prevention, diagnosis, and treatment of chronic hepatitis B AASLD 18 hepatitis B guidance Hepatology 18 Apr 67 (4)Feb;71(2) doi /hep Authors Marc G Ghany 1 , Timothy R Morgan 2 , AASLDIDSA Hepatitis C Guidance Panel
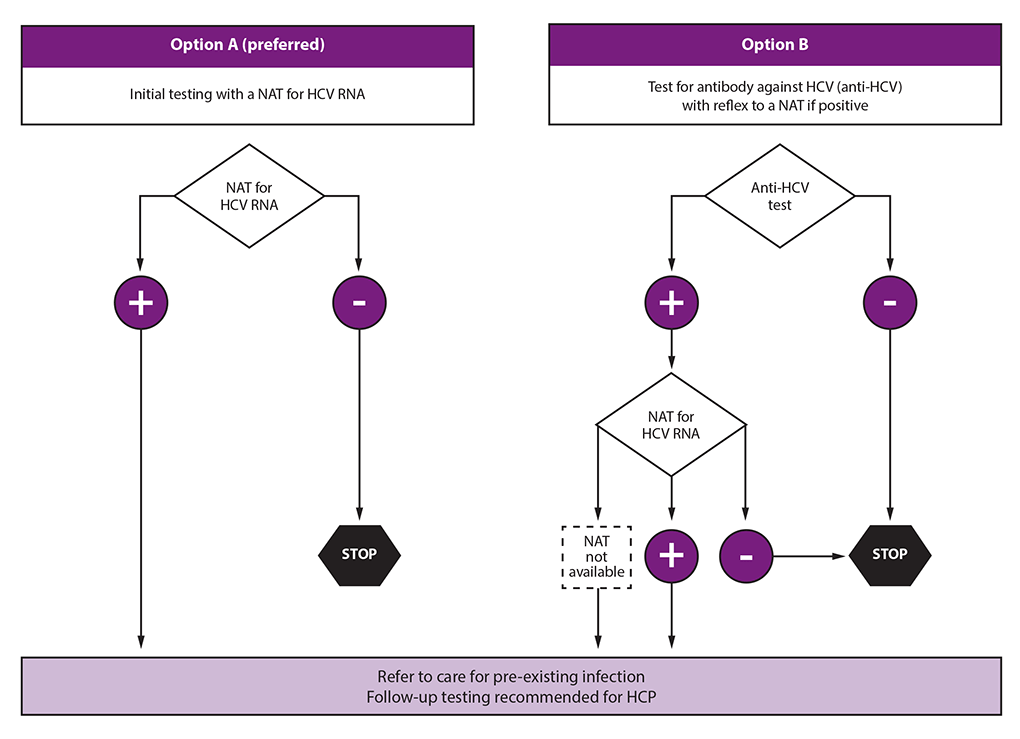



Testing And Clinical Management Of Health Care Personnel Potentially Exposed To Hepatitis C Virus Cdc Guidance United States Mmwr
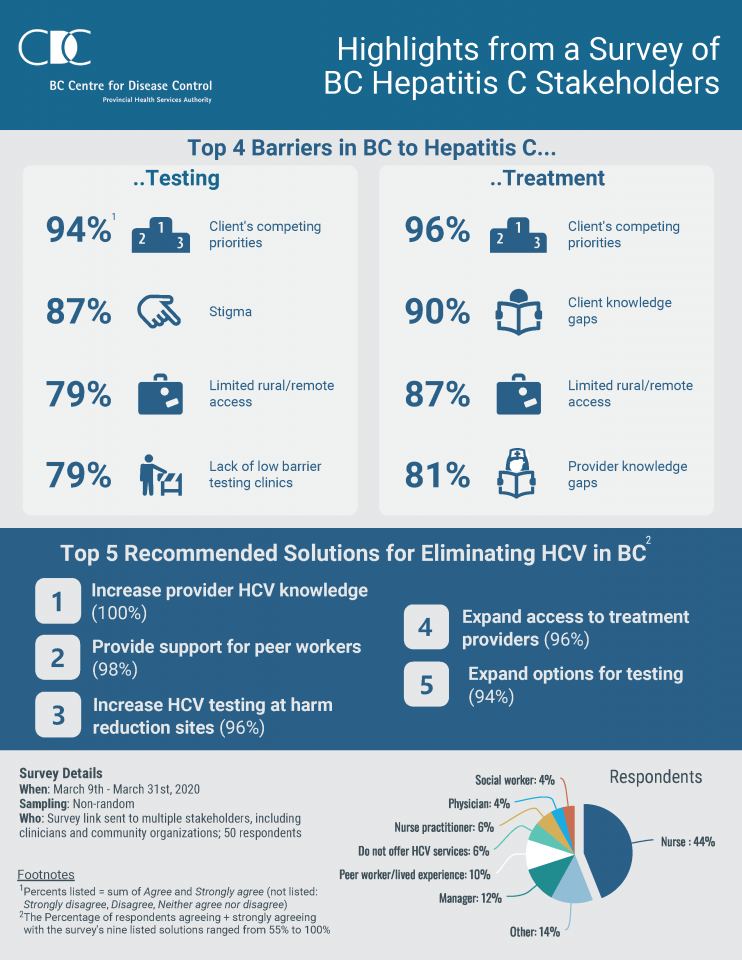



Hepatitis Education Canada Programme Canadien D Education Sur L Hepatite
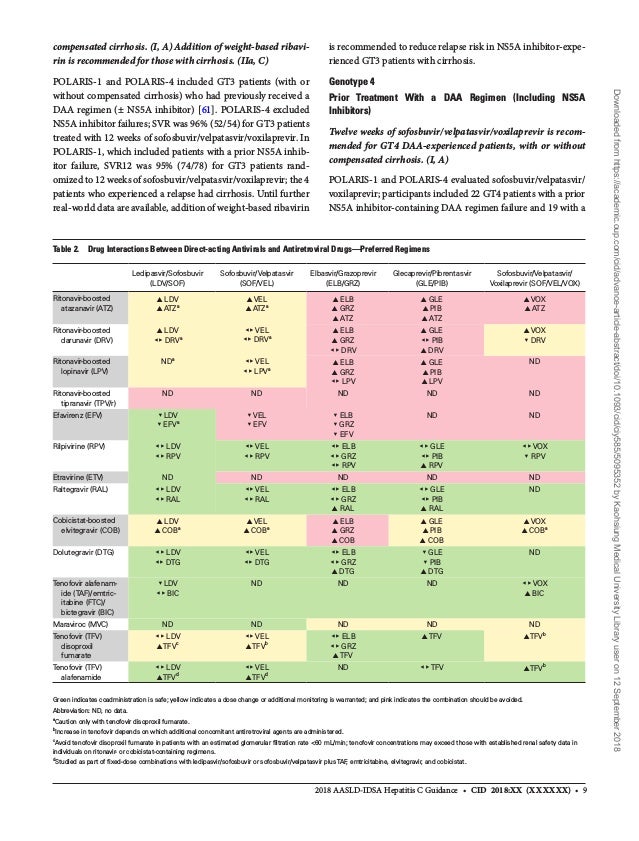
This section provides guidance on the retreatment of persons with chronic HCV infection in whom prior therapy failed The level of the evidence available to inform the best regimen for each patient and the strength of the recommendation vary and are rated accordingly (see Methods Table 2)In addition, specific recommendations are given when treatment differs for a particular group (eg,CDC Recommendations for HCV Screening On April 10, , the Centers for Disease Control and Prevention (CDC) issued new recommendations for hepatitis C screening among adults in the United States (Table 1)1 This new guidance augments prior CDC guidance on HCV screening with two new major recommendations (1) all adults aged 18 yearsACTG Presents Data Showing Minimal Monitoring Approach to Hepatitis C Treatment is Safe and Successful at AASLD's Liver Meeting the hepatitis C virus is not detected by standard blood




Hepatitis C The Lancet
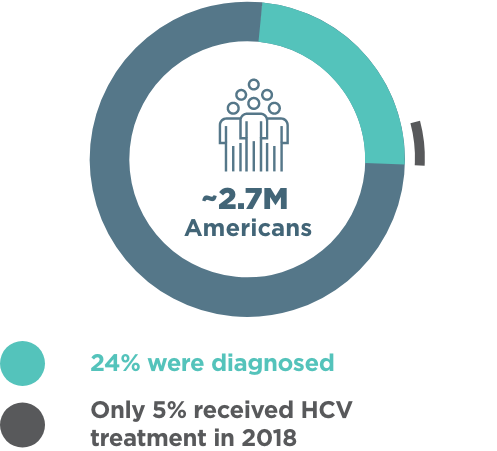



About Hepatitis C Hcv Official Hcp Site
Summary Exposure to hepatitis viruses is a recognized occupational risk for health care personnel (HCP) This report establishes new CDC guidance that includes recommendations for a testing algorithm and clinical management for HCP with potential occupational exposure to hepatitis C virus (HCV)Purpose and Scope The objectives of this document are to provide guidance in the diagnosis and management of autoimmune hepatitis (AIH) based on current evidence and expert opinion and to present guidelines to clinically relevant questions based on systematic reviews of the literature and the quality of evidence 1 This practice guideline/guidance constitutes an update of the guidelinesBurnout in the Pandemic Name It, Frame It and Tame It Emerging Topic Conference Chronic Hepatitis B From the Population to New Molecules and Back



English World Gastroenterology Organisation
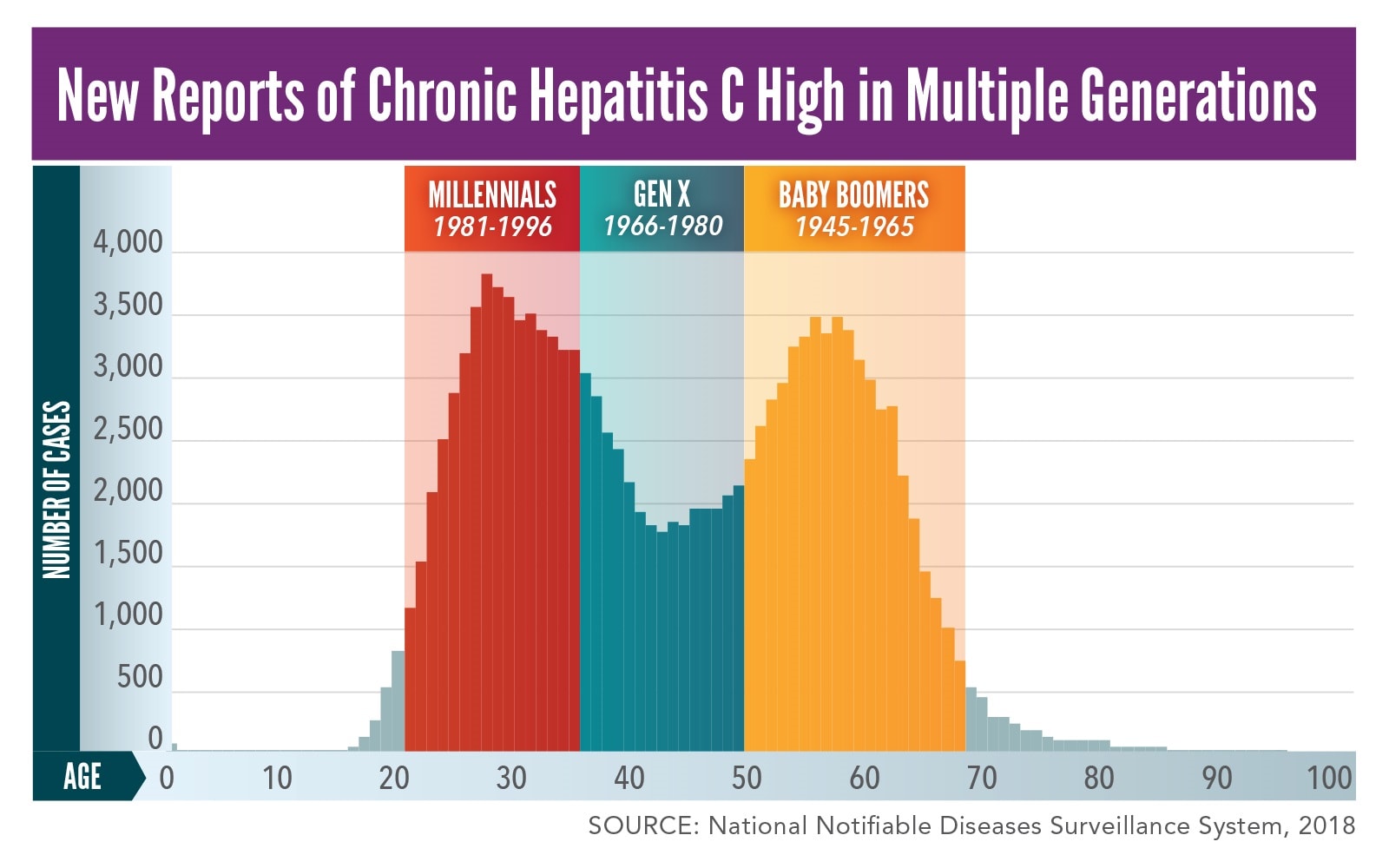



Cdc Vital Signs Dramatic Increases In Hepatitis C Cdc
PRACTICE GUIDANCE Hepatology, Vol 71, No 2, Hepatitis C Guidance 19 Update American Association for the Study of Liver Diseases–Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection AASLDIDSA Hepatitis C Guidance Panel* T he American Association for the Study ofWebinar Hepatitis C Infection Beyond the Liver 48 (5 votes) Recorded On and is member of the updating panels of the clinical practice guidelines for HCV of the EASL and of the World Health Organisation Harrys A Torres Harrys A Torres, MD, FACP, FIDSA, is an Associate Professor of Medicine in the Department ofAPASL Guidelines for HCV "APASL consensus statements and recommendation for hepatitis C prevention, epidemiology, and laboratory testing" (Hepatol Int 16 –701) *This APASL Guideline can be downloaded by clicking below



1




Cost Burden Of Hepatitis C Virus Treatment In Commercially Insured Patients
October 6, The AASLD congratulates Drs Harvey J Alter, Michael Houghton, and Charles M Rice, the recipients of the Nobel Prize in Physiology or Medicine Their groundbreaking research identified the Hepatitis C virus (HCV) – a bloodborne pathogen that causes acute and chronic hepatitis, cirrhosis, and liver cancerTable 1 Summary of the Process and Methods for the Guidance Development Table 2 Rating System Used to Rate Level of Evidence and Strength of Recommendation Table 3 Commonly Used Abbreviations and Their Expansions References Testing, Evaluation, and Monitoring of Hepatitis C Browse Topics Testing, Evaluation, and Monitoring of Hepatitis CMedia Contacts Nola Gruneisen, AASLD, 571‐292‐3068 Lauren Martin, IDSA, () HCVguidelinesorg — a website developed by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America to provide uptodate guidance on the management of hepatitis C — was recently revised to reflect important



Q Tbn And9gcsljbhdpiznbj9k6l8qqtgjqahya4jugfwbozhd1joe9dt66uf4 Usqp Cau



Medicine Utah Edu Students Programs Md Curriculum Ruute Pdfs Project Echo New Mexico Pdf
EASL recommendations on treatment of hepatitis C Final update of the seriesq European Association for the Study of the Liver* Summary Hepatitis C virus (HCV) infection is a major cause of chronic liver disease, with approximately 71 million chronically infected individuals worldwide Clinical care for patients with HCVrelatedThis treatment is a breakthrough for all HBV carriers Reply Delete Replies18 years old but is recommended for unaccompanied refugee minors




One World One Pandemic Many Guidelines Management Of Liver Diseases During Covid 19 Gut
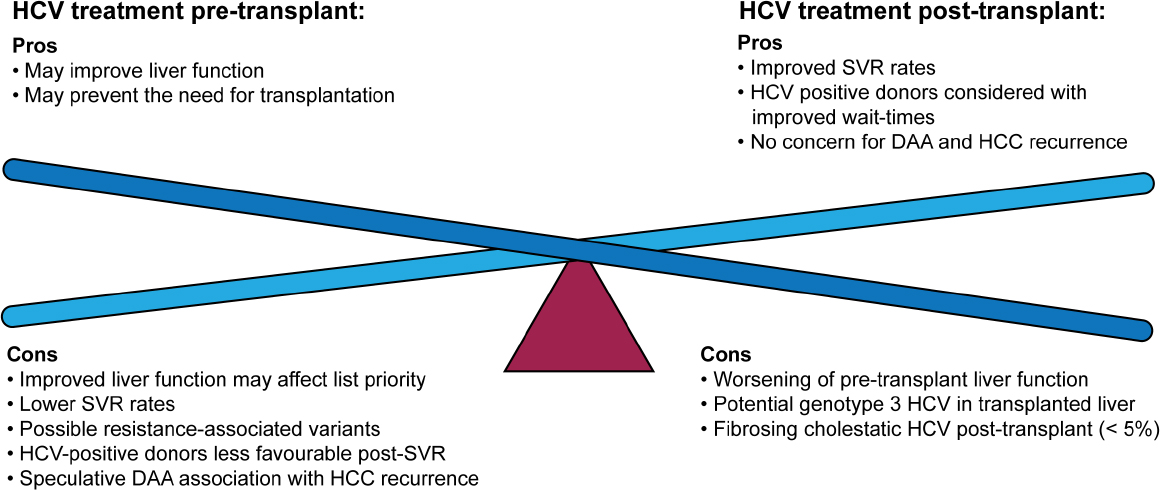



Management Of Concomitant Hepatocellular Carcinoma And Chronic Hepatitis C A Review
This AASLD 18 Hepatitis B Guidance is intended to complement the AASLD 16 Practice Guidelines for Treatment of Chronic Hepatitis B(1) and update the previous hepatitis B virus (HBV) guidelines from 09 The 18 updated guidance on chronic hepatitis B (CHB) includes (1) updates on treatment since the 16 HBV guidelines (notably theTreatment Policy for the Management of Chronic Hepatitis C Updated and Effective March 30, This policy was developed by the California Department of Health Care Services (DHCS) based upon a review of the medical literature, the most recent guidelines, and reports published by the American Association for the Study of Liver Diseases (AASLD)/HCV = hepatitis C virus;




Hepatitis B Related Outcomes Following Direct Acting Antiviral Therapy In Taiwanese Patients With Chronic Hbv Hcv Co Infection Sciencedirect
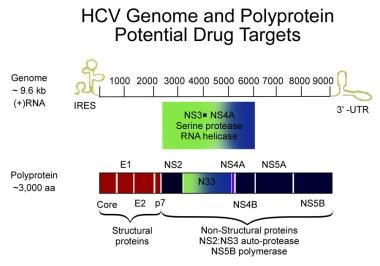



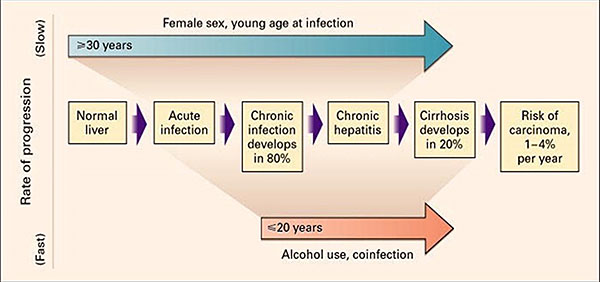
Hepatitis C Practice Essentials Background Pathophysiology
Based on scientific evidence and expert opinion, recommendations are rated by the level of evidence (I, II, or III) and the strength of the recommendation (A, B, or C) using a system adapted from the American College of Cardiology and the American Heart Association 5, 6 See the original AASLD–IDSA hepatitis C guidance publication 7 or theRecommendations • Patients with cirrhosis, those with autoimmune hepatitis on immunosuppressive medications, and posttransplant patients on immunosuppressant therapy are potentially at increased risk forRecommendations for testing, managing, and treating hepatitis C Updated August 27, AASLD/IDSA HCV guidance panel Recommendations for testing, managing, and treating hepatitis C Updated August 27, Internet cited August 27, http//hcvguidelinesorg/



sld Guidelines Hepatitis C




Cdc Recommendations For Hepatitis C Screening Among Adults United States Mmwr
"We started the online hepatitis C guidance, just two of us, Dave Thomas from the Infectious Disease Society of America and myself, back in 12, early 13 when we knew that the number of new medications that were hitting the market were going to overwhelm the old ability of our guidelines process to handle it, so we started an online processThe American Association for the Study of Liver Diseases, along with other societies, has updated guidelines for hepatitis C treatment, including the use of directacting antiviral drugsAs of January , the CDC, USPSTF, and the American Association for the Study of Liver Disease and Infectious Disease Society of America updated hepatitis C testing guidelines to reflect universal testing of all adults at least once, and pregnant women with each pregnancy (AASLD, 18;




Hepatitis C Guidance 19 Update American Association For The Study Of Liver Diseases Infectious Diseases Society Of America Recommendations For Testing Managing And Treating Hepatitis C Virus Infection Ghany




sld Idsa Recommendations For Patients Who Would Receive The Most Download Table
HCP = health care personnel;Diagnosis and Management of Autoimmune Hepatitis in Adults and Children 19 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases Cara L Mack, David Adams, David N Assis, Nanda Kerkar, Michael P Manns, Marlyn J Mayo, John M Vierling, Mouaz Alsawas, Mohammad H Murad, Albert J Czaja , HepatologyThe following is an outline and list of Core Competency Modules (with subcompetency lessons and learning objective performance indicators) Module 1 Screening and Diagnosis of Hepatitis C Infection Module Core Competency Apply EvidenceBased Recommendations to Provide Screening and Diagnosis of HCV Infection



Core Concepts Screening And Diagnosis Of Hepatitis C Infection Hepatitis C Online




Hcv Hepatitis C Virus
TreatmentNaive Genotype 3 The following pages include guidance for management of treatmentnaive patients with genotype 3 infection TreatmentNaive Genotype 3 Without Cirrhosis TreatmentNaive Genotype 3 With Compensated Cirrhosis Simplified HCV Treatment for TreatmentNaive Adults Without Cirrhosis Last update August 27,The American Liver Foundation was created to serve as a public education and patient information resource Visit their website or call 1800GOLIVER () for more liver information and resources Viral Hepatitis in the US – The Road to Elimination An Update from HHS/CDC at AASLD's TLMdXGuidelines for testing persons with HIV CDC guidelines for testing persons born , "Baby Boomer" birth cohort 1999 18 IDSA/AASLD guidelines for testing during pregnancy IDSA, Infectious Diseases Society of America AASLD, American Association for the Study of Liver Diseases US Strategy to End the Hepatitis C Epidemic 4




New Idsa sld Guidelines For Hepatitis C Youtube



1



Dhhr Wv Gov Bms Bms pharmacy Documents Hepatitis c criteria v 1a Pdf



Www Ccah Alliance Org Providerspdfs Hcv Checklist Pdf




Pdf Easl Clinical Practice Guidelines Management Of Hepatitis C Virus Infection




Efficacy And Safety Of Ravidasvir Plus Sofosbuvir In Patients With Chronic Hepatitis C Infection Without Cirrhosis Or With Compensated Cirrhosis Storm C 1 Interim Analysis Of A Two Stage Open Label Multicentre Single Arm Phase 2 3




Practice Guidelines sld




Comparison Of The Current International Guidelines On The Management Of Hcc Sciencedirect




Hepatitis C Guidance 19 Update American Association For The Study Of Liver Diseases Infectious Diseases Society Of America Recommendations For Testing Managing And Treating Hepatitis C Virus Infection Ghany




Recommendations For Testing Managing And Treating Hepatitis C Hcv Guidance




Summary Of sld Idsa And Easl Guidelines For Management Of Download Table




Updated Pathway To Micro Elimination Of Hepatitis C Virus In The Hemodialysis Population Kidney International Reports



Quest Diagnostics Hep C Screening And Diagnosis



Www sld Org Sites Default Files 19 06 Hbvguidance Terrault Et Al 18 Hepatology Pdf




The Hepatitis C Virus Care Cascade In The New York City Jail System During The Direct Acting Antiviral Treatment Era 14 17 Eclinicalmedicine




Hcc Surveillance After Svr In Patients With F3 F4 Fibrosis Journal Of Hepatology




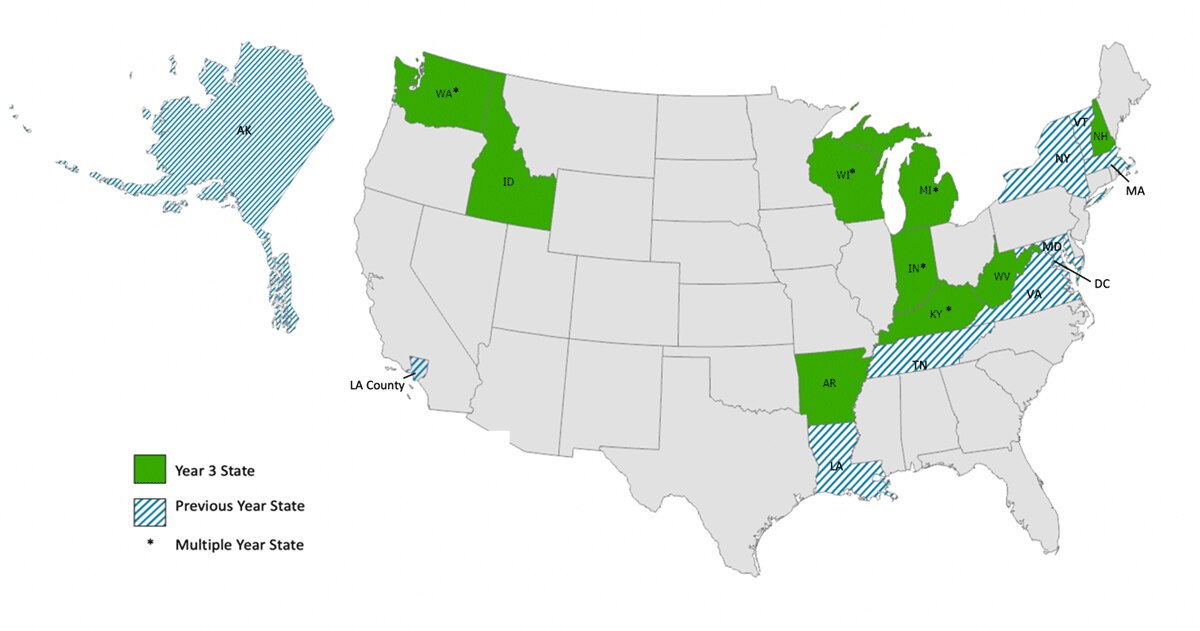
Estimating The Year Each State In The United States Will Achieve The World Health Organization S Elimination Targets For Hepatitis C Springerlink




Ledipasvir Gutsandgrowth
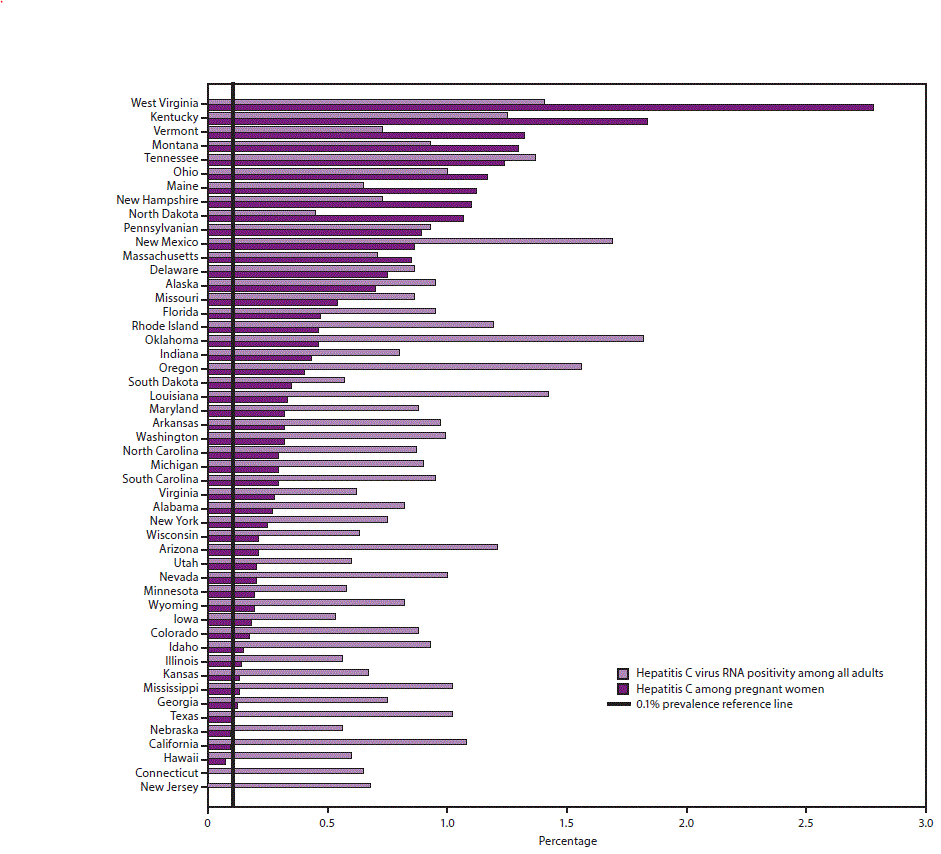



Cdc Recommendations For Hepatitis C Screening Among Adults United States Mmwr




Pdf Hepatitis C Guidance 18 Update sld Idsa Recommendations For Testing Managing And Treating Hepatitis C Virus Infection




Utilization Of Hepatitis C Virus Infected Organ Donors In Cardiothoracic Transplantation An Ishlt Expert Consensus Statement The Journal Of Heart And Lung Transplantation




Practice Guidelines sld



Hepatitis C Chapter 4 Yellow Book Travelers Health Cdc
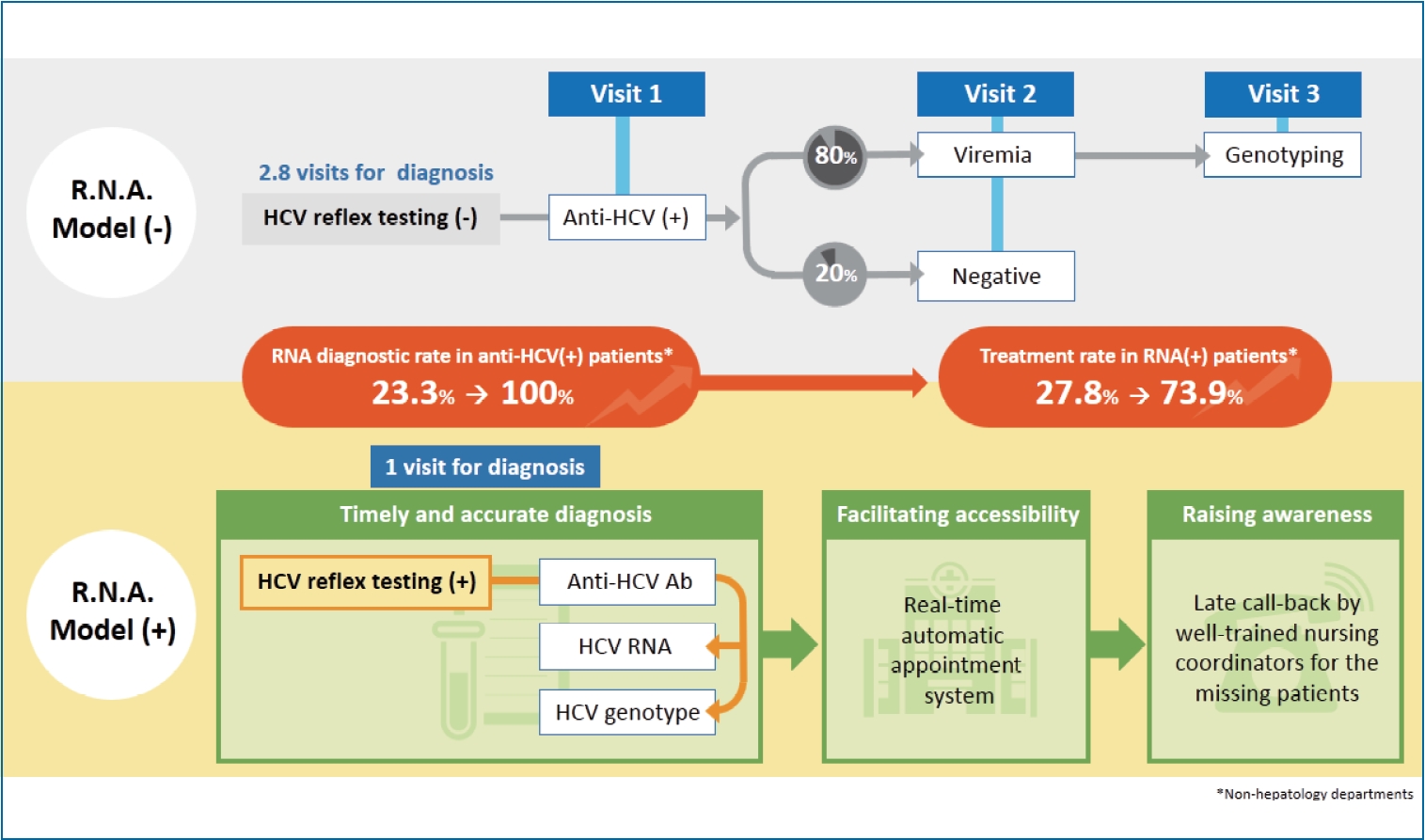



Scaling Up The In Hospital Hepatitis C Virus Care Cascade In Taiwan



Www sld Org Sites Default Files 19 06 sld 18 Hcc Guidance On Diagnosis 2c Staging And Management Hep 281 29 Pdf




Hepatitis C Guidance 19 Update American Association For The Study Of Liver Diseases Infectious Diseases Society Of America Recommendations For Testing Managing And Treating Hepatitis C Virus Infection Ghany




Limitations Of Non Invasive Tests For Assessment Of Liver Fibrosis Jhep Reports




An Educate Test And Treat Model Towards Elimination Of Hepatitis C Infection In Egypt Feasibility And Effectiveness In 73 Villages Journal Of Hepatology




Where Are The Children In National Hepatitis C Policies A Global Review Of National Strategic Plans And Guidelines Jhep Reports




Hepatitis C Wikipedia




Abbreviations sld American Association For The Study Of Liver Download Scientific Diagram




Hepatitis C In Naspghan Position Paper Gutsandgrowth




Core Concepts Treatment Of Hcv Genotype 3 Treatment Of Chronic Hepatitis C Infection Hepatitis C Online




18 Kidney Disease Improving Global Outcomes Kdigo Hepatitis C In Chronic Kidney Disease Guideline Implementation Asia Summit Conference Report Kidney International Reports
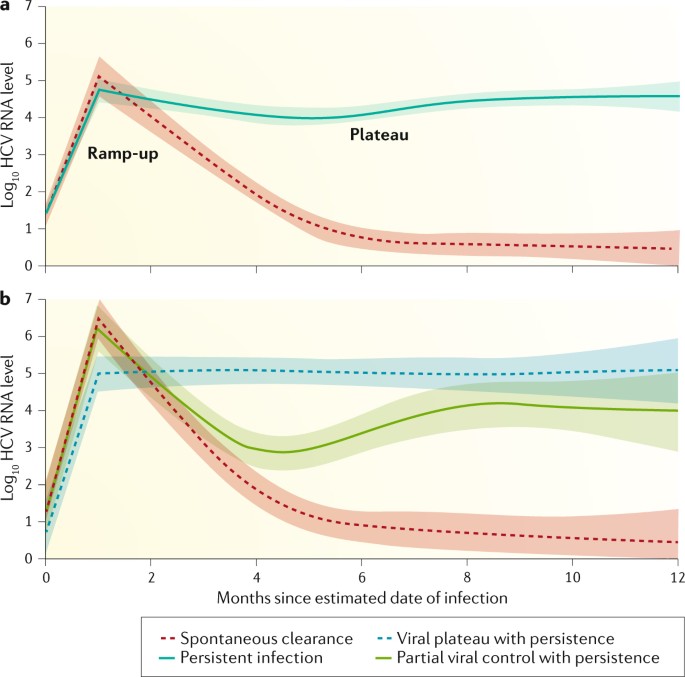



Management Of Acute Hcv Infection In The Era Of Direct Acting Antiviral Therapy Nature Reviews Gastroenterology Hepatology



1




Recommendations For Testing Managing And Treating Hepatitis C Hcv Guidance
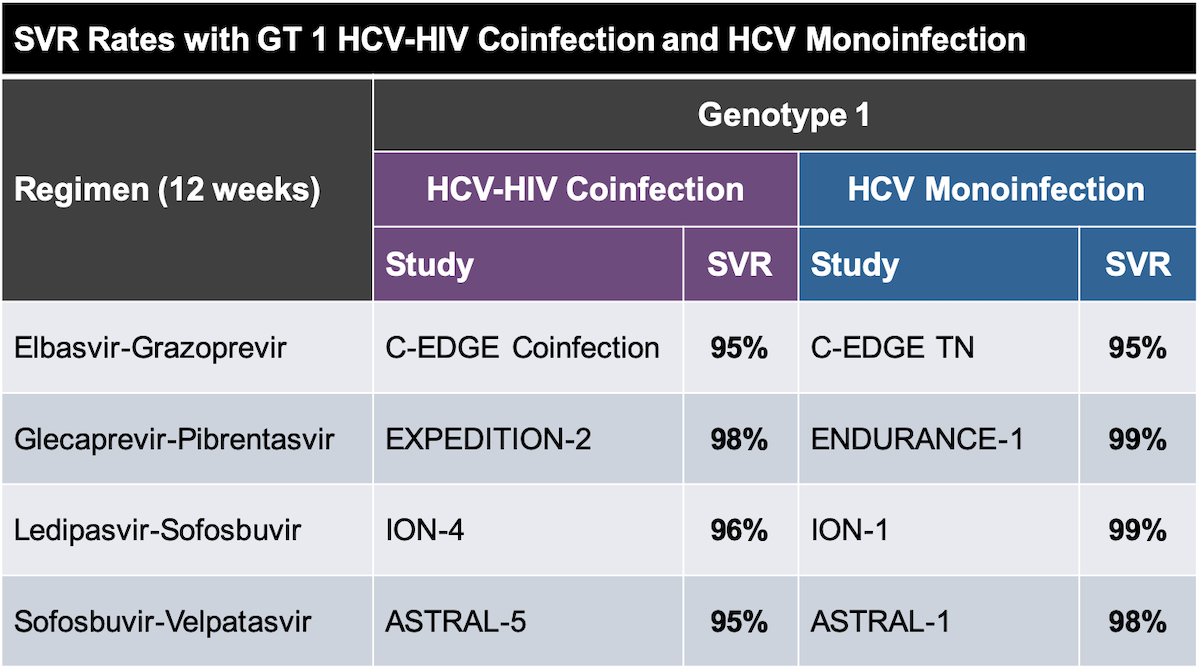



Core Concepts Treatment Of Hcv In Persons With Hiv Coinfection Treatment Of Key Populations And Unique Situations Hepatitis C Online




Kdoqi Us Commentary On The 18 Kdigo Clinical Practice Guideline For The Prevention Diagnosis Evaluation And Treatment Of Hepatitis C American Journal Of Kidney Diseases




Hepatitis B Related Outcomes Following Direct Acting Antiviral Therapy In Taiwanese Patients With Chronic Hbv Hcv Co Infection Journal Of Hepatology




Hepatitis C In Chronic Kidney Disease An Overview Of The Kdigo Guideline Clinical Gastroenterology And Hepatology



Low Adherence To Infant Hcv Testing Guidelines Among Pregnant Women With Hcv Cirrhosis




Treatment Of Hepatitis C A New Paradigm Toward Viral Eradication The Journal Of Pediatrics
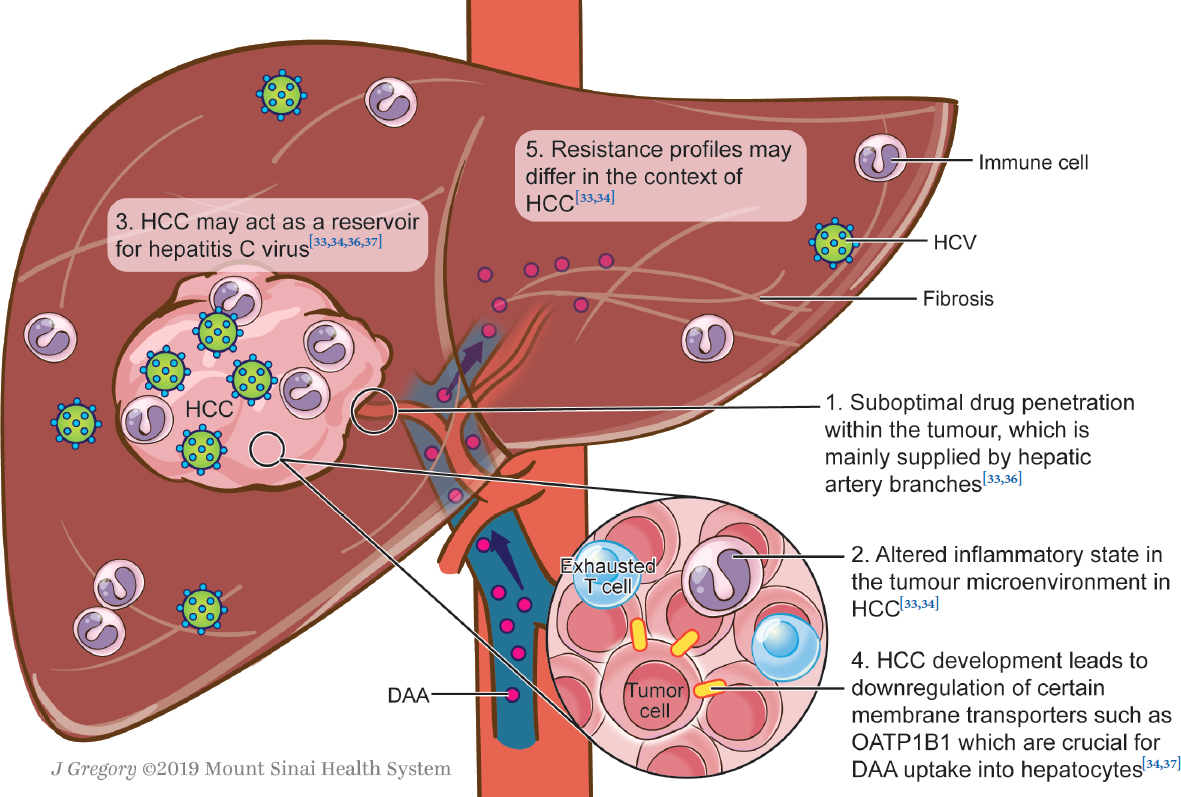



Management Of Concomitant Hepatocellular Carcinoma And Chronic Hepatitis C A Review




Diagnosis And Management Of Hepatitis C American Family Physician




Hepatitis C In Chronic Kidney Disease An Overview Of The Kdigo Guideline Clinical Gastroenterology And Hepatology




sld Recommendations For Treatment Of Chronic Hepatitis C Download Table



2



Www Hcvguidelines Org Printpdf Node 654 Page Pdf File 2 Testing and linkage




Practice Guidelines sld




Hcv Screening Guidelines Hcv In Women Module Hcv In Women Hepatitis Clinical Care Options



Hepatitis C Medicaid Affinity Group Hhs Gov



Hepatitis C Cleveland Clinic




Easl 17 Clinical Practice Guidelines On The Management Of Hepatitis B Virus Infection Journal Of Hepatology




Hepatitis C Guidance 19 Update American Association For The Study Of Liver Diseases Infectious Diseases Society Of America Recommendations For Testing Managing And Treating Hepatitis C Virus Infection Ghany




Hepatitis C Cancer Therapy Advisor
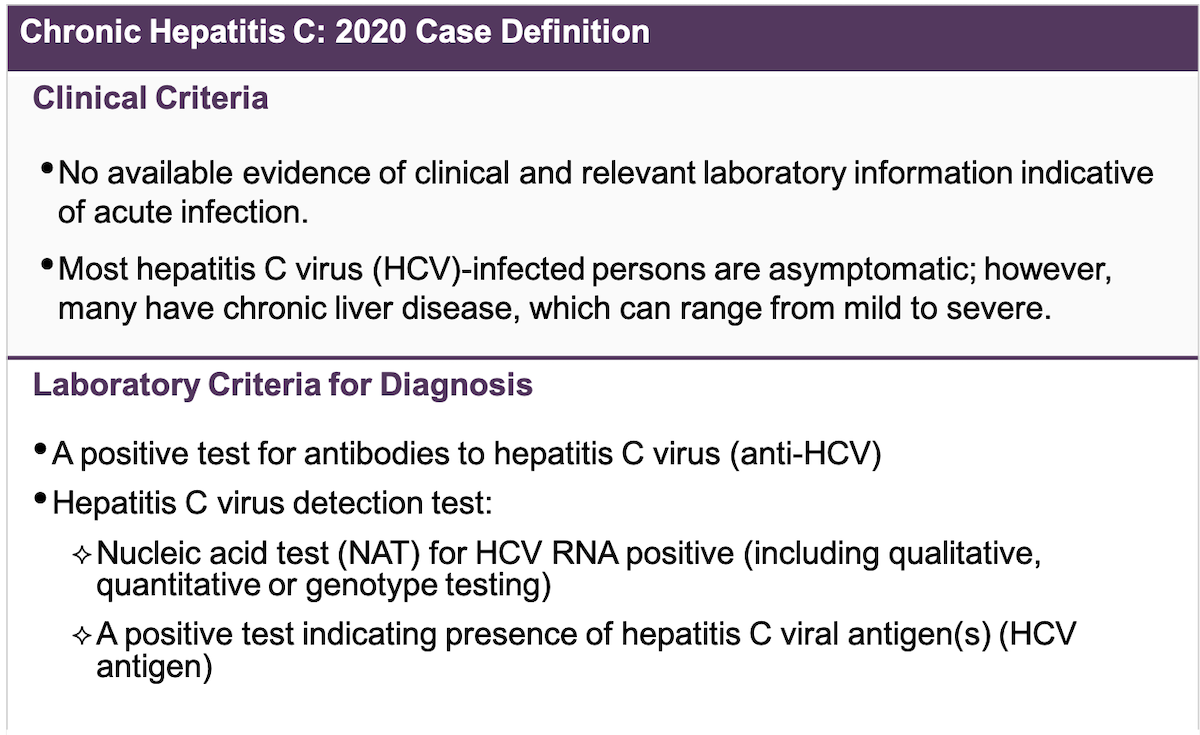



Core Concepts Screening And Diagnosis Of Hepatitis C Infection Hepatitis C Online




Successful Hepatitis C Treatment Reduces Risk Of Liver Cancer And Death But Most Remain Untreated Aidsmap




The Liver Meeting Hepatitis Sessions Coalition For Global Hepatitis Elimination
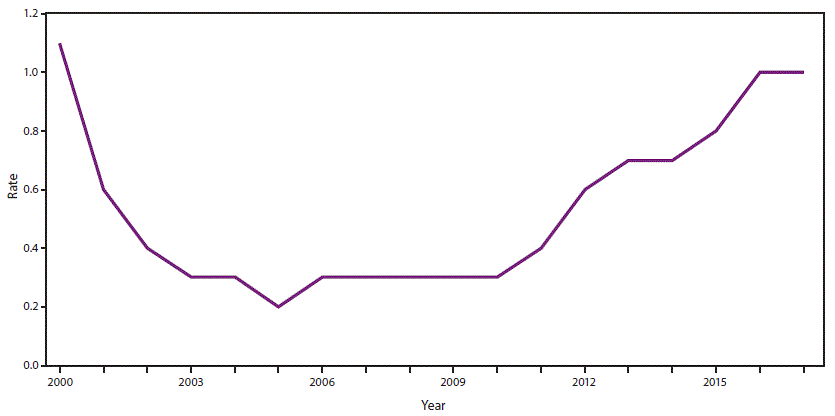



Cdc Recommendations For Hepatitis C Screening Among Adults United States Mmwr




Hepcx Hep Free Nyc




Hepatitis C Global Epidemiology And Strategies For Control Clinical Microbiology And Infection
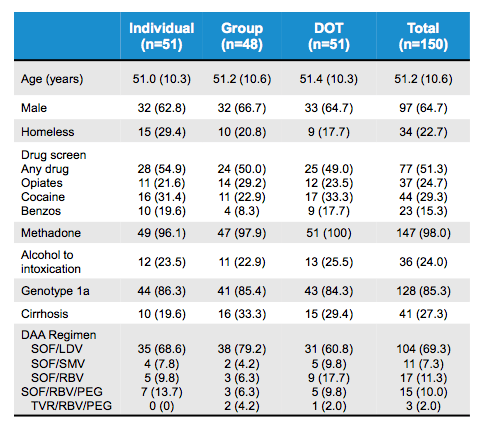



Summary From Easl 17 For Hepatitis C Global Implementation Of Hepatitis C Hcv Treatment What Are The Successes What Are The Remaining Challenges




sld Updates Chronic Hepatitis B Recommendations Practice Guidelines American Family Physician
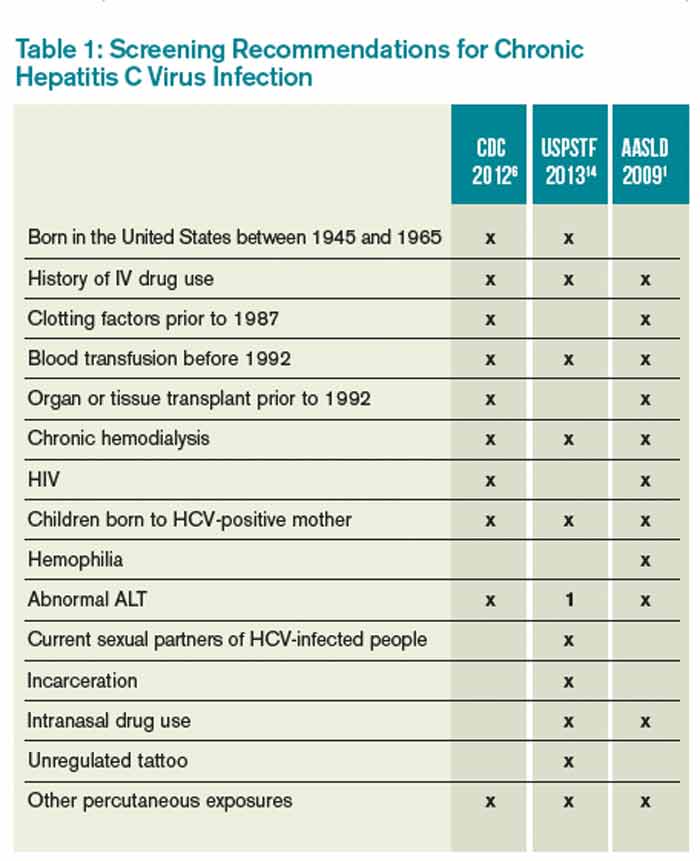



Which Patients Should Be Screened For Hepatitis C Virus Infection The Hospitalist
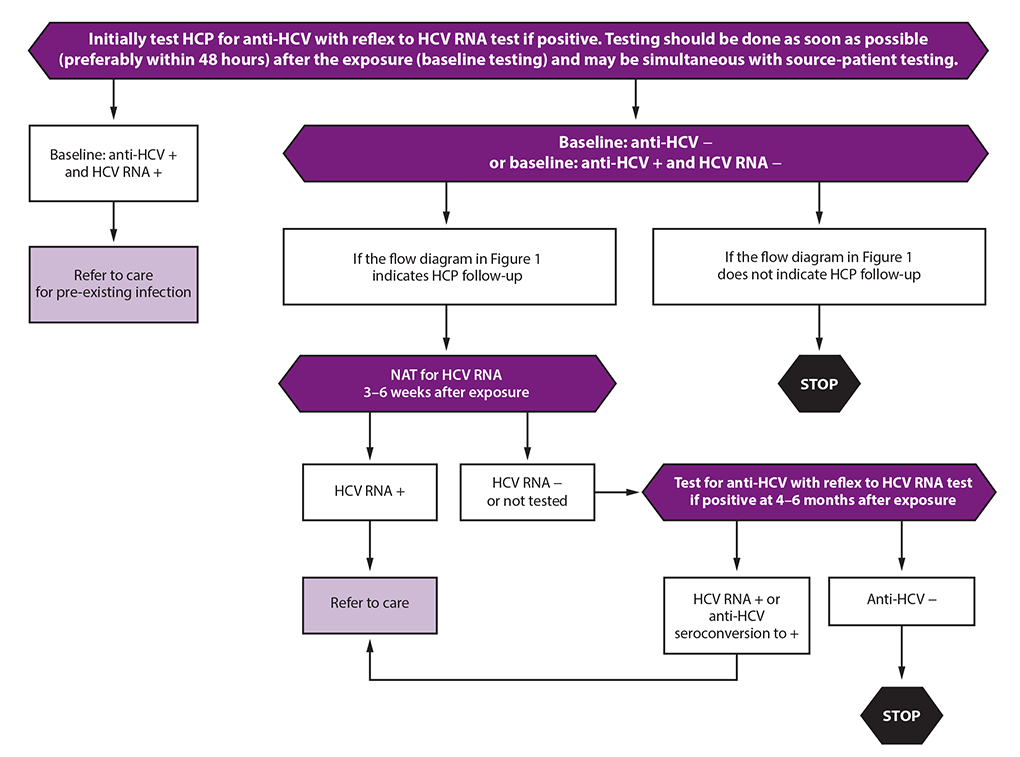



Testing And Clinical Management Of Health Care Personnel Potentially Exposed To Hepatitis C Virus Cdc Guidance United States Mmwr



Advances In Hepatitis C Treatment
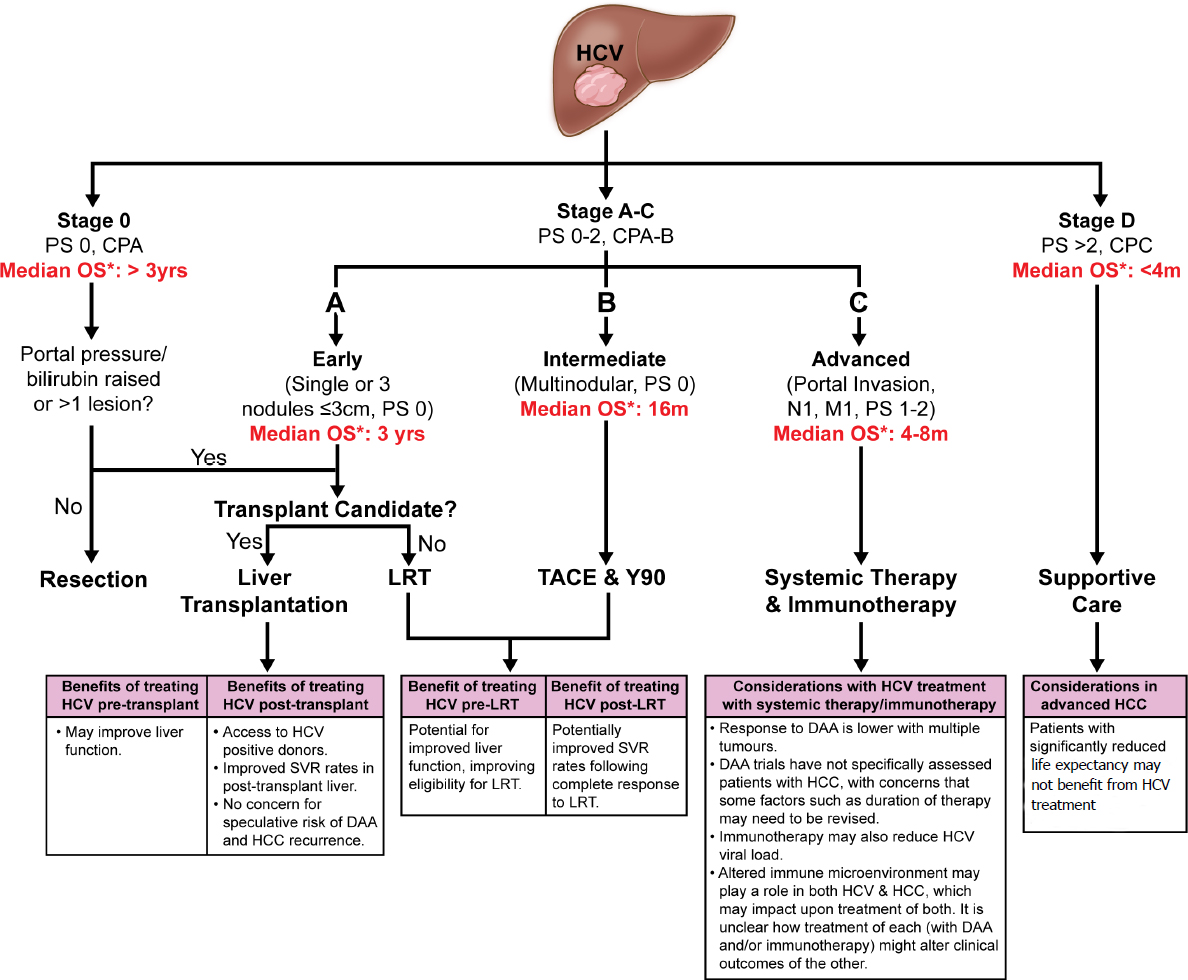



Management Of Concomitant Hepatocellular Carcinoma And Chronic Hepatitis C A Review




Hepatitis C Guidance 19 Update American Association For The Study Of Liver Diseases Infectious Diseases Society Of America Recommendations For Testing Managing And Treating Hepatitis C Virus Infection Ghany




Hcv Screening Guidelines Hcv In Women Module Hcv In Women Hepatitis Clinical Care Options




Hepatitis C Virus Gutsandgrowth
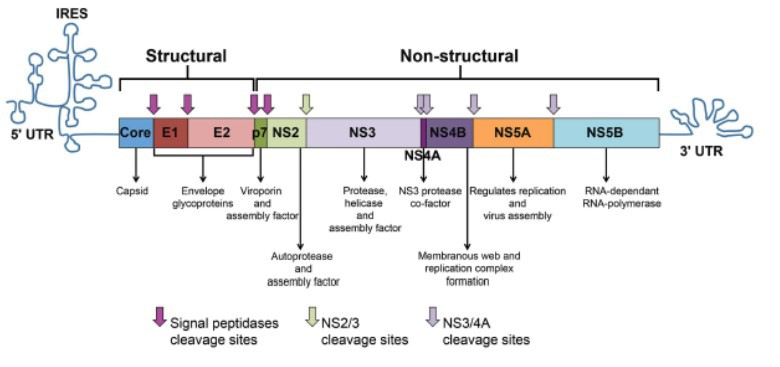



Hepatitis C And Chronic Kidney Disease Overview Of Evaluation And Management National Kidney Foundation




Viral Hepatitis Elimination Call To Action sld




Guidance For Design And Endpoints Of Clinical Trials In Chronic Hepatitis B Report From The 19 Easl sld Hbv Treatment Endpoints Conference Journal Of Hepatology




Multicenter Study To Transplant Hepatitis C Infected Kidneys Mythic An Open Label Study Of Combined Glecaprevir And Pibrentasvir To Treat Recipients Of Transplanted Kidneys From Deceased Donors With Hepatitis C Virus Infection American




Easl 17 Clinical Practice Guidelines On The Management Of Hepatitis B Virus Infection Journal Of Hepatology


